Discovery Chemistry Services

Chemistry-Led Drug Discovery, Built for Confident Progression
At PI Health Sciences, our Drug discovery chemistry services form the execution backbone of early drug discovery, translating biological hypotheses into chemically viable, development-ready lead series. We support hit identification and hit-to-lead programs with a sharp focus on scientific rigor, speed, and early risk reduction, ensuring chemistry decisions actively enable downstream success rather than defer complexity.
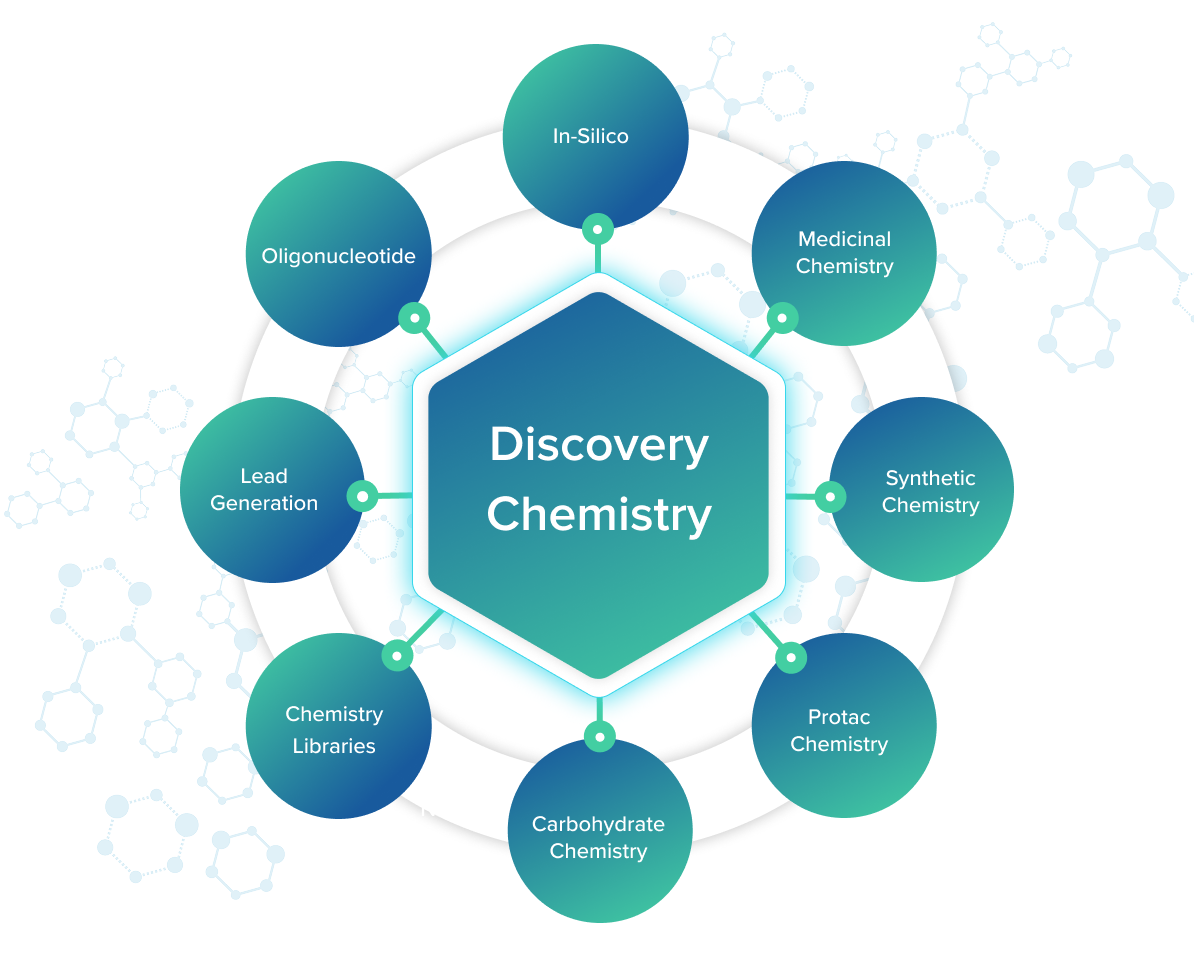
Our drug discovery chemistry services are designed around a chemistry-led operating philosophy, where molecular design, synthesis, and iteration are tightly integrated with biological feedback, DMPK insights, and early liability awareness. The goal is not compound volume, but rapid convergence on chemically robust, optimizable series that can progress into preclinical development with confidence.
By delivering Integrated discovery chemistry services under a single scientific framework, PI Health Sciences enables coherent decision-making across discovery functions. Chemistry, biology, and in silico intelligence operate as one continuous loop, ensuring that potency, selectivity, physicochemical properties, and feasibility are evaluated together from the outset.
In-Silico
In-Silico Discovery at PI Health Sciences
We deliver in-silico discovery as a decision-support discipline embedded within early drug discovery chemistry. Our work focuses on the computational evaluation, prioritization, and risk assessment of chemical matter from hit generation through hit-to-lead optimization, helping teams focus chemistry on the most viable molecular directions. In-silico methods are applied as a filtering and prioritization layer, working alongside experimental validation to reduce uncertainty rather than introduce false confidence.
How Our Approach Is Different
In-silico discovery at PI Health Sciences is not treated as a one-time virtual screening exercise. Computational insights are applied iteratively within active discovery programs, continuously refined using experimental feedback. All predictions are reviewed in the context of chemical intuition, biological relevance, and downstream feasibility, ensuring that computational outputs directly support medicinal chemistry decision-making.
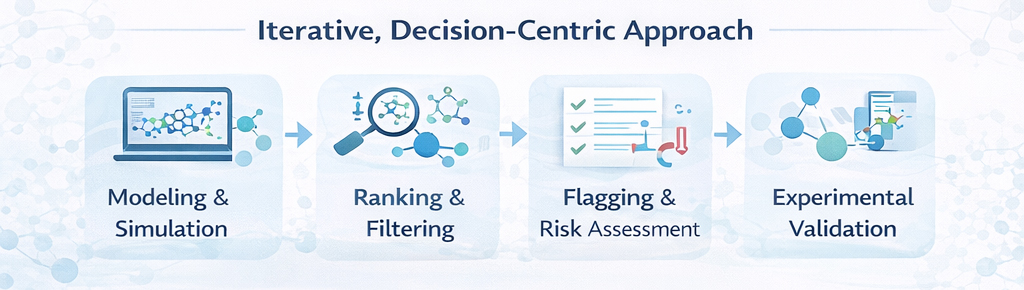
Core Design Principles
- Use of in-silico models to compare and prioritize compounds, not to over-optimize predictions
- Emphasis on relative ranking rather than absolute prediction values
- Early flagging of structural alerts, reactive functionalities, and potential liabilities
- Preference for interpretable outputs that chemists can act on confidently
- Continuous alignment with medicinal chemistry workflows and experimental data
Talk to Our Experts
Medicinal Chemistry
Chemistry-Led Molecular Design for Confident Discovery Decisions
Medicinal Chemistry at PI Health Sciences is the core of our Discovery Chemistry capability, where biological hypotheses are translated into rational small-molecule designs. We design, synthesize, and optimize chemical matter for hit identification and hit-to-lead programs, with a clear focus on chemical robustness, biological relevance, and early decision confidence. Our objective is rapid convergence on viable chemical series that justify continued investment, not synthetic throughput without direction.
How Our Approach Is Different
Our medicinal chemistry approach is chemistry-led and decision-centric. Design, synthesis, and optimization are tightly integrated with biology, DMPK, and in-silico insights to guide programs early and decisively. Medicinal chemistry is treated as a scientific decision-making discipline, where every structural change is evaluated for biological impact, chemical liability, and future development feasibility.
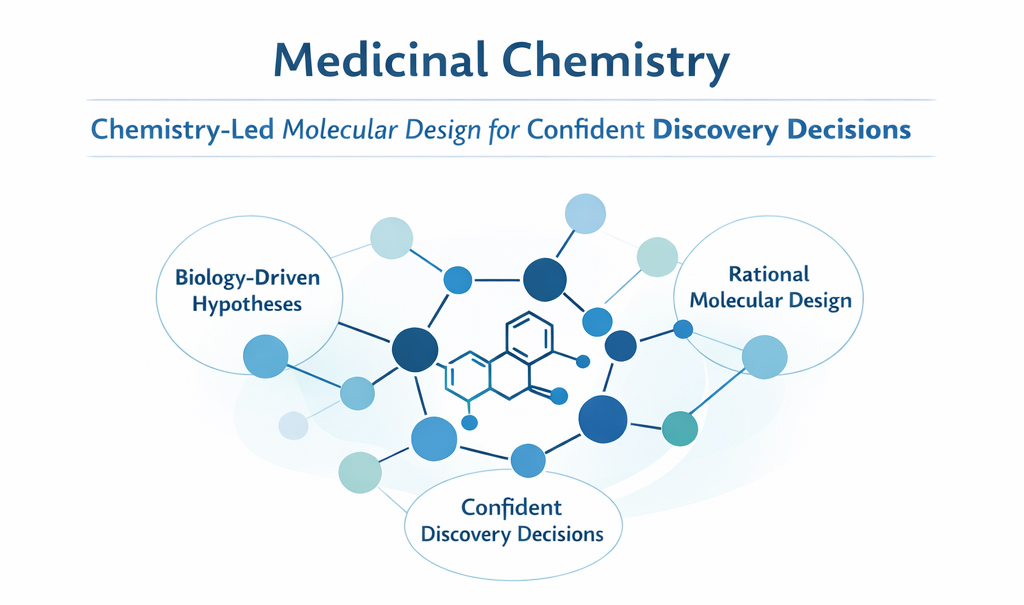
Core Design Principles
- Molecular design guided by SAR trends and biological feedback
- Strategic scaffold selection, expansion, and refinement
- Optimization of potency, selectivity, and physicochemical properties
- Early identification and mitigation of chemical liabilities
- Synthetic feasibility assessed from the earliest design cycles
- Prioritization of chemical series with sustainable optimization pathways
Talk to Our Experts
Synthetic Chemistry
Precision synthesis that enables confident discovery decisions
At PI Health Sciences, Synthetic Chemistry Services serves as the execution backbone of discovery programs, translating molecular design hypotheses into real, testable chemical matter with speed, reproducibility, and structural integrity. Our synthetic chemistry capabilities are purpose-built to support hit identification and hit-to-lead programs, where rapid iteration and chemically reliable compounds are essential for early-stage decision-making. We approach synthetic chemistry as a decision-enabling discipline rather than a transactional synthesis function, ensuring that early chemistry supports, rather than constrains, downstream discovery progress.
How Our Approach Is Different
Our synthetic chemistry strategy is grounded in feasibility, reproducibility, and future robustness from the outset. Every synthetic route, reaction condition, and intermediate choice is evaluated not only for speed of execution, but also for impurity risk, reaction stability, and scalability awareness. Rather than optimizing chemistry for one-off success, we focus on chemistry that works consistently, allowing promising chemical matter to progress without later setbacks caused by avoidable synthetic fragility. In silico tools are used to prioritize and de-risk chemistry, such as narrowing chemical space and flagging potential liabilities. All computational outputs are critically reviewed by chemists and used to inform experimental design, not replace it.

Core Design Principles
- Route-first, feasibility-aware synthetic planning
- Preference for robust, reproducible chemical transformations
- Early identification and control of impurity-forming reactions
- Minimization of step count and unnecessary protecting group usage
- Selection of reagents and conditions aligned with safety and scalability considerations
- Continuous refinement of routes based on experimental performance
Talk to Our Experts
Protac Chemistry
Protac Chemistry-Led Risk Mitigation
Protac Chemistry at PI Health Sciences focuses on the deliberate chemical modification of molecular structures to proactively address known or emerging liabilities during drug discovery. The service is designed to mitigate toxicity signals, metabolic instability, reactivity risks, and developability concerns while preserving core biological activity. By embedding risk-aware chemical decisions early, Protac Chemistry supports confident progression through hit-to-lead and lead optimization stages, reducing the likelihood of avoidable late-stage failures.
How Our Approach Is Different
Protac Chemistry is treated as a strategic, decision-driven discipline, not a corrective step applied after problems escalate. Every structural modification is guided by a clear liability hypothesis, informed by biological data, DMPK feedback, or in silico risk assessment. Chemical changes are purposefully designed, experimentally validated, and continuously evaluated against downstream feasibility, ensuring that risk mitigation is scientifically justified rather than cosmetic.
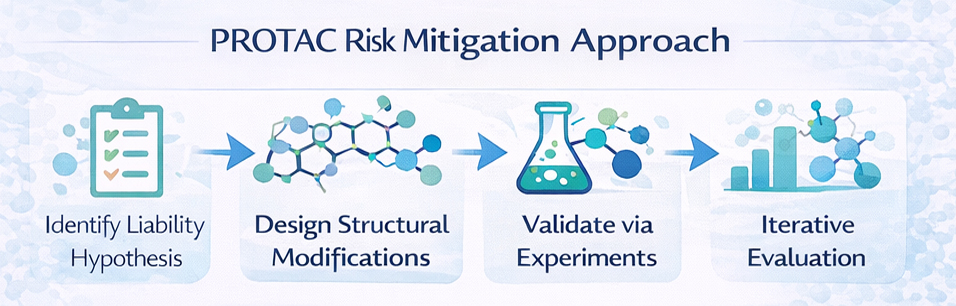
Core Design Principles
- Targeted modification of liability-driving structural motifs
- Preservation of core pharmacophore and target engagement
- Avoidance of substitutions that introduce new safety or reactivity risks
- Consideration of metabolic pathways and chemical stability
- Integration of DMPK and biological feedback into design decisions
- Preference for chemically stable and developable analogs
Talk to Our Experts
Carbohydrate Chemistry
Precision Synthesis of Structurally Defined Carbohydrate Matter
Carbohydrate Chemistry at PI Health Sciences delivers specialized synthetic and modification capabilities for carbohydrate-containing molecules used in drug discovery programs. Our work focuses on the controlled design, synthesis, and functionalization of monosaccharides, oligosaccharides, glycoconjugates, and carbohydrate-derived motifs that play critical roles in biological recognition and mechanism of action. By enabling chemically well-defined carbohydrate structures, we support hypothesis-driven discovery, target engagement studies, and early structure-activity exploration.
How Our Approach Is Different
Carbohydrate Chemistry is executed as a high-discipline scientific function, not routine synthesis. Every stereochemical outcome, linkage position, and protecting group choice is deliberately controlled to ensure reproducibility, interpretability, and downstream usability. Our structure-first approach prioritizes stereochemical fidelity and linkage control, allowing biological outcomes to be confidently attributed to defined structural features rather than synthetic variability.
Core Design Principles
- Explicit control of anomeric configuration and stereochemistry
- Rational protecting group strategies to guide selectivity
- Strict linkage fidelity and structural definition
- Minimization of batch-to-batch synthetic variability
- Avoidance of unstable or poorly characterized carbohydrate motifs
- Design compatibility with biological, biophysical, and analytical evaluation
Talk to Our Experts
Chemistry Libraries
Purpose-Built Libraries for Insight-Driven Discovery
Chemistry Libraries at PI Health Sciences are designed as strategic discovery assets, not volume-driven compound inventories. Our libraries are purpose-built to enable rapid biological hypothesis testing, efficient SAR generation, and early decision confidence across hit identification and hit-to-lead programs. Each library is assembled with clear scientific intent, balancing chemical diversity, developability awareness, and biological relevance to ensure that every compound synthesized contributes meaningful learning to discovery progression.
How Our Approach Is Different
Rather than maximizing compound count, we prioritize structural quality, annotation depth, and experimental usability. Chemistry Libraries are treated as decision-enabling infrastructure, designed to generate insight rather than noise. Library composition is guided by defined biological or chemical objectives, ensuring chemical coherence and downstream readiness as discovery priorities evolve.
Core Design Principles
- Purpose-driven library design aligned to defined discovery objectives
- Balanced exploration of chemical diversity and focus
- Preference for drug-like and lead-like chemical space
- Avoidance of structurally unstable or reactive motifs
- Consistent scaffold integrity across library members
- Design compatibility with downstream optimization workflows
Talk to Our Experts
Lead Generation
Lead Generation in Discovery Design
At PI Health Sciences, our Lead Generation in Discovery Design platform enables efficient and risk-balanced drug discovery through an integrated combination of molecular design, translational biology, and computational intelligence. Programs are initiated with a clear understanding of target biology, chemical feasibility, developability, and IP landscape, ensuring that lead generation decisions are intentional, efficient, and aligned with downstream success. Our integrated model treats hit identification, lead generation, and optimization as a continuous process rather than sequential handoffs, allowing discovery campaigns to progress with clarity, precision, and regulatory awareness from the earliest stages.
How Our Approach Is Different
Our approach to lead generation is grounded in early risk balancing and strategy integration. Rather than optimizing hits in isolation, we design lead generation strategies that embed developability, translational relevance, and IP considerations from the outset. This reduces rework, accelerates progression, and increases the likelihood that generated leads can advance efficiently toward preclinical development.
Core Design Principles
- Integrated assessment of target biology, chemical tractability, and modality suitability
- Early incorporation of developability, IP strategy, and translational relevance
- AI-enabled hit discovery combined with chemistry-led feasibility assessment
- Scaffold expansion strategies that preserve molecular recognition while enabling novelty
- Knowledge-driven SAR design informed by literature and patent intelligence
- Continuous, generation-centric execution across discovery stages
Talk to Our Experts
Oligonucleotide
Targeted Gene Modulation for Confident Discovery and Development Decisions
Oligonucleotide therapeutics at PI Health Sciences focus on the rational design of nucleic acid-based molecules
that modulate gene expression with precision. We support antisense oligonucleotides, siRNA, and related
constructs designed to interact directly with RNA targets, enabling selective control of biological pathways
that are difficult to address with small molecules.
Our approach emphasizes early clarity in sequence selection, chemistry choices, and biological intent. By
evaluating these elements together, we help teams avoid advancing oligonucleotide designs that appear promising
early but later fail due to instability, off-target effects, or limited development potential.
How Our Approach Is Different
Our oligonucleotide work is design-led and discovery-driven. Sequence design is guided by target biology and
mechanism, while chemistry choices are made with a clear view of stability, delivery, and experimental
practicality. Rather than treating oligonucleotides as sequences alone, we treat them as chemical entities that
must perform reliably in real biological systems.
Computational analysis, chemical insight, and biological context are integrated from the start, allowing early
refinement of sequences before committing to extensive synthesis or testing.
Core Design Principles
- Mechanism-driven selection of target RNA regions
- Rational choice between antisense and RNA interference strategies
- Use of chemical modifications to improve stability and performance
- Early consideration of off-target binding and immune activation risk
- Design choices informed by synthesis and handling practicality
- Focus on sequences that can be optimized without repeated redesign
Talk to Our Experts
Capabilities

Hit Identification and Early Chemistry Support
Chemistry support for hit identification programs, including rapid synthesis of hit matter and early structure, activity trend evaluation to enable confident progression within Discovery chemistry services.

Hit-to-Lead Optimization
Iterative hit-to-lead programs focused on refining potency, selectivity, and physicochemical properties through tight design-synthesis-data feedback cycles supporting drug discovery chemistry services.
Medicinal Chemistry Design and SAR Development
Medicinal chemistry-driven molecular design supported by systematic SAR development to guide rational optimization and early decision-making.
Scaffold Identification and Optimization
Identification, expansion, and optimization of core scaffolds with clear structure–property relationships and defined optimization trajectories, avoiding chemically fragile series early.
Synthetic Route Design and Feasibility Assessment
Design of efficient, reproducible synthetic routes with early evaluation of robustness, impurity risk, and step economy, aligned with expectations for mature CRO chemistry services.
Knowledge-Driven Handoff to Preclinical Chemistry
Clear documentation of design rationale, SAR interpretation, and synthetic reproducibility to ensure seamless transition into preclinical development without downstream rework.
Frequently asked questions
We’re here to help with any questions you have about our plans, supported features, and how our model works.
What stages of drug discovery are covered under Discovery Chemistry services at PI Health Sciences?
PI Health Sciences supports discovery programs from hit identification through hit-to-lead optimization. Discovery Chemistry activities typically conclude once a lead series demonstrates sufficient chemical robustness, SAR clarity, and readiness for transition into preclinical development.
What types of molecules are supported within Discovery Chemistry programs?
Our Discovery Chemistry services support a broad range of chemical modalities, including small molecules, PROTACs, carbohydrate-based compounds, and custom chemistry libraries. Molecular strategies are selected based on target biology, tractability, and downstream development feasibility.
How does PI Health Sciences ensure chemistry decisions are development-ready?
Chemistry decisions are made with early consideration of synthetic feasibility, impurity risk, physicochemical properties, and scalability. By embedding development awareness into discovery chemistry, PI Health Sciences minimizes downstream rework and reduces late-stage attrition risk.
How are scaffolds identified and optimized during discovery chemistry?
Scaffold identification and optimization are guided by chemical tractability, SAR depth, and optimization headroom. PI Health Sciences prioritizes scaffolds with clear expansion potential and defined optimization trajectories to support efficient progression into lead optimization.
How are synthetic routes designed during Discovery Chemistry?
Synthetic routes are designed with reproducibility, robustness, and step economy in mind. Early evaluation of impurity risks, raw material availability, and route scalability ensures chemistry outputs meet expectations for mature CRO and preclinical chemistry programs.
Contact Us
Connect with PI Health Sciences to advance your discovery chemistry services, from hit identification and hit-to-lead optimization to high-quality lead generation grounded in scientific rigor.
