Integrated Discovery Biology Services

Mechanism-Driven Biology, Built for Confident Discovery Progression
PI Health Sciences Discovery Biology services offers comprehensive screening across the full spectrum of preclinical integrated drug discovery. Supported by advanced scientific expertise and infrastructure, we provide end-to-end solutions spanning major disease areas, therapeutic modalities, and early development needs.
Our capabilities cover molecular target validation, structural characterization, advanced biochemical and cellular screening, ADME, pharmacokinetics profiling, and in vivo evaluation through partner sites, enabling decision-ready progression from discovery to lead selection.
PI Health Sciences supports discovery programs across a wide range of therapeutic modalities, including small molecules, peptides, engineered biologics, and emerging drug formats. Our integrated in vitro platforms enable rapid screening, potency assessment, and mechanistic profiling to accelerate lead identification and optimization.
We also deliver specialized Discovery Biology workflows for complex large molecules, including monoclonal antibodies (mAbs), antibody-drug conjugates (ADCs), bi-specifics, and peptide-based therapeutics. Through integrated platforms assessing binding, affinity, internalization, and immune-mediated functional activity, we generate robust outputs such as dose-response relationships and IC₅₀ values, supporting rapid advancement of high-quality candidates.
Core Services
Structural Biology
With the advances in genomics, the high-throughput methods of structure determination can not only provide powerful approaches to accelerate lead identification and optimization, but also lead discovery. Our protein scientists synthesize crystallographic grade recombinant protein and co-crystal structures with small-molecule compounds to support structure-based drug discovery (SBDD). We have established collaborations with global synchrotrons (eg: Australian Synchroton) cryo-EM facilities to use the three-dimensional protein structure, as a basis for drug discovery and lead optimisation.
Protein production and crystallisation services
- Gene cloning, plasmid preparation and stable cell line generation
- Protein expression and protein purification for in vitro assay and crystallization
- Protein crystallization of targets.
- Crystal structure determination and generation of small molecule
- complexes with protein through soaking and/or co-crystallization
Talk to Our Experts
Molecular Biology
PI Health Sciences Discovery Services provides both prokaryotic and mammalian custom engineered cell line services, starting from plasmid construction to protein expression verification. We are proficient in a variety of cutting-edge methods such Plasmid/Lentiviral/Adenoviral/BACMAM vector based stable and transient transfections, electroporation and lipid nanoparticle-based transfections. We have a timeline of 4-5 weeks for a stable cell pool and 10-12 weeks for a stable cell line.
Plasmid DNA and Molecular Cloning
PI Health Sciences provides end-to-end plasmid design and molecular cloning services, including transformation workflows, competent cell preparation, and bacterial culture expansion. We support both blunt-end and sticky-end cloning strategies, alongside mutagenesis and advanced sequence- and ligation-free cloning approaches. Our systems are optimized across standard bacterial platforms such as DH5α, Stbl3, and JM109 to enable reliable construct generation for downstream discovery applications.
Transcript Expression
Our transcript expression capabilities include high-quality RNA extraction from diverse sample types, including cell lines, fresh and frozen tissues, and FFPE specimens. We perform quantitative gene expression analysis using both SYBR Green and TaqMan real-time PCR methodologies, supporting copy number determination and relative expression profiling to enable robust target validation and pathway confirmation.
Protein Expression and Quantification
PI Health Sciences delivers comprehensive protein expression verification and quantification using orthogonal analytical platforms, including native and SDS-PAGE, Western blotting and JESS systems, ELISA, FACS, and Biacore-based interaction analysis. Additional capabilities such as co-immunoprecipitation support mechanistic protein studies and functional characterization across discovery-stage workflows.
Cell Engineering
We offer advanced cell engineering services through plasmid, lentiviral, adenoviral, and BACMAM vector-based stable and transient transfection systems. Our platforms include electroporation and lipid nanoparticle-based delivery, reporter gene promoter assays, IRES-enabled multi-protein expression, and siRNA/shRNA knockdown models. We also support tagged and organelle-specific protein expression (Golgi, ER, nucleus, membrane) to enable precise functional interrogation in disease-relevant cellular contexts.
Talk to Our Experts
Target Identification and Validation
PI Health Sciences Discovery Biology provides integrated Target Identification and Validation services to support early discovery programs from hypothesis generation through target confirmation. With advances in functional genomics, systems biology, and translational disease models, we enable the systematic identification of high-confidence therapeutic targets linked directly to disease biology. Our scientific platforms establish target relevance, mechanism of action, and biological tractability, ensuring that targets are robust, druggable, and aligned with downstream development strategies across therapeutic modalities.
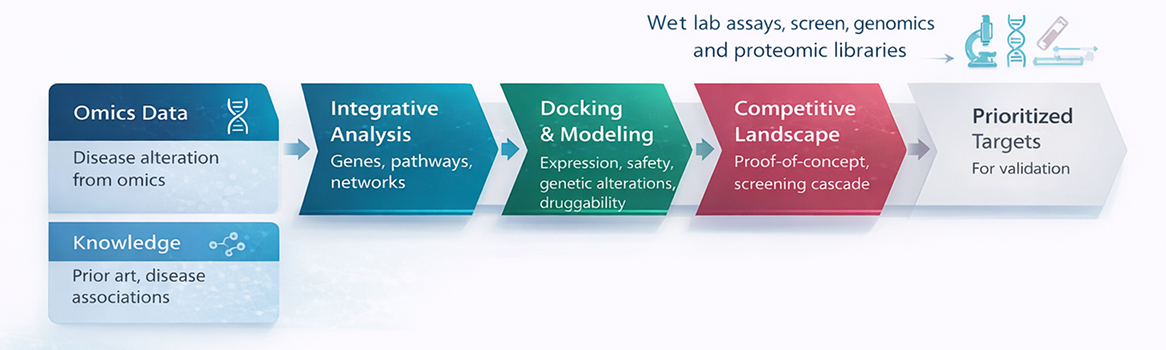
Target discovery and validation services include:
Target Selection & Characterization
PI Health Sciences supports systematic target prioritization through biological characterization, druggability assessment, and disease relevance confirmation. Our integrated discovery frameworks enable confident selection of high-value targets aligned with therapeutic strategy and downstream development feasibility.
Disease Mechanisms
We investigate disease-driving mechanisms through functional biology and pathway-linked validation assays. These studies establish the causal role of targets within disease progression and provide mechanistic evidence to guide early discovery decisions.
Model Selection and Assay Planning
PI Health Sciences designs fit-for-purpose cellular and molecular assay systems using disease-relevant models optimized for target interrogation. Our scientists ensure assay strategy, biological context, and readouts are aligned to support robust validation and screening workflows.
Omics (Genomics)
Our genomics-enabled discovery platforms support target identification through integrated omics analysis and functional genomics validation. These approaches enable systematic mapping of target-disease associations and prioritization of biologically actionable candidates.
Pathway Analysis
PI Health Sciences delivers pathway-driven validation through mechanistic profiling of signaling networks and downstream biomarker response. We confirm target involvement, pathway impact, and functional modulation to strengthen translational confidence.
Knock-in/Knock-out Studies
We deliver mechanistic target validation through complementary genetic perturbation platforms, including CRISPR-based knock-in/knock-out modulation and RNA interference approaches. These workflows confirm target dependency, pathway consequence, and functional impact in cellular systems, providing decision-ready evidence for progression into hit identification and lead optimization.
Talk to Our Experts
In Vitro Biology Assays & Instrumentation
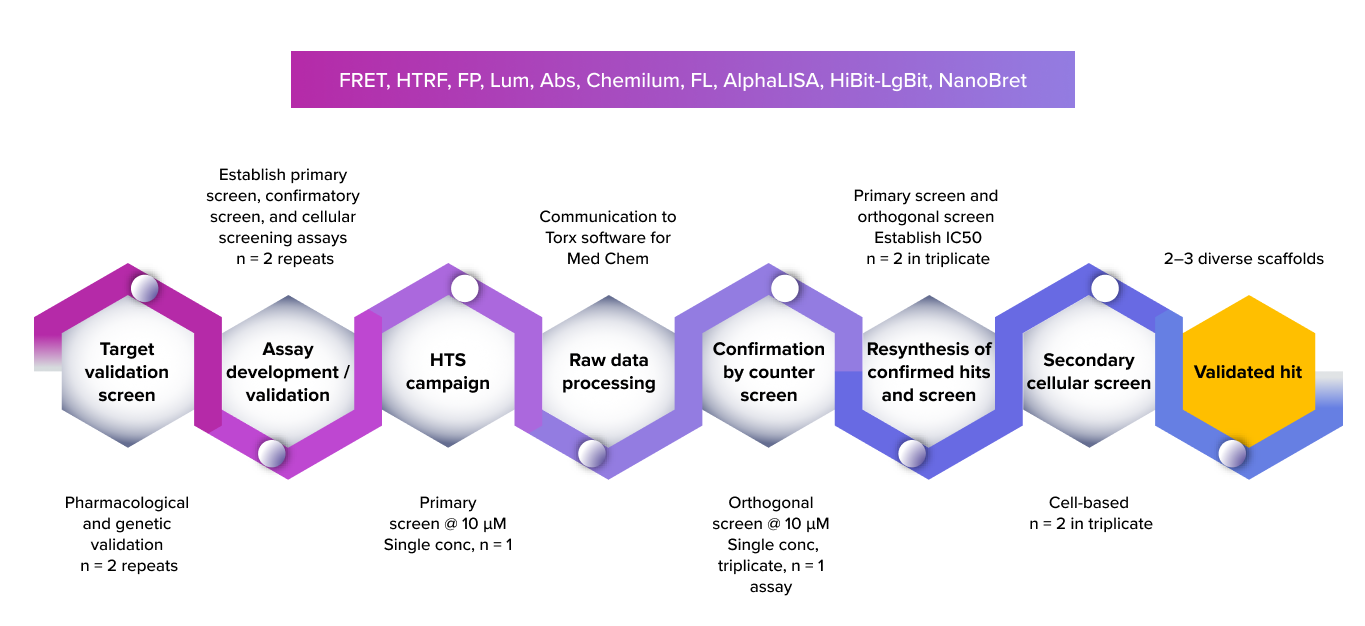
At PI Health Sciences , our in vitro screening and biochemical assay platforms provide the experimental backbone for integrated drug discovery across small molecules, peptides, and biologics, including monoclonal antibodies. With advanced assay technologies and scalable screening formats, we enable rapid identification of active compounds, early potency confirmation, and mechanistic characterization to support confident progression through discovery pipelines.
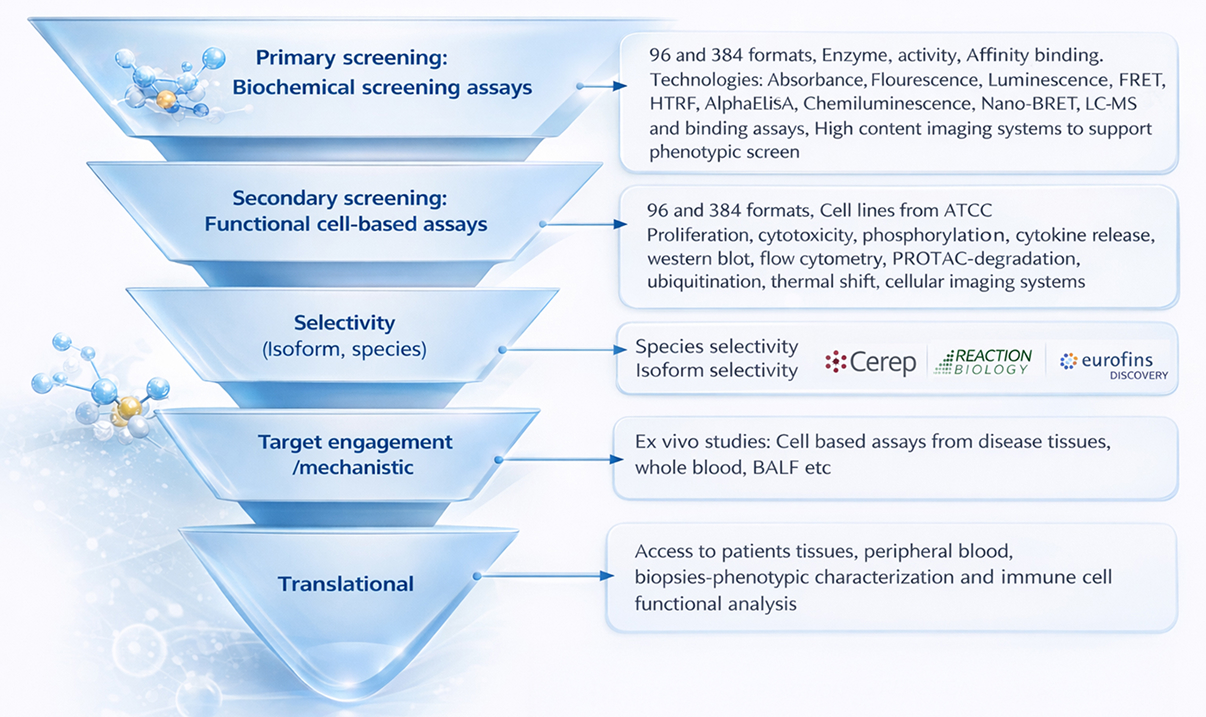
An end-to-end drug discovery screening workflow—from primary biochemical assays to cell-based functional screening, selectivity profiling, target engagement, and translational studies—designed to validate hits and advance them toward clinical relevance.
Our biochemical screening capabilities span enzyme activity, binding, and dose-response profiling using
multiple
detection platforms, including fluorescence intensity, time-resolved fluorescence (HTRF), luminescence,
absorbance, fluorescence polarization, AlphaLISA, and calcium flux assays. These assays generate robust
outputs
such as percent inhibition, IC₅₀ values, and full dose-response curves in medium to high-throughput
formats.
PI Health Sciences also offers quantitative SPR-based interaction analysis using high-throughput Biacore
systems to
evaluate
peptide-protein, protein-protein, antigen-antibody, and small molecule-protein interactions. These
studies
provide key kinetic and affinity parameters, including k_on, k_off, and K_D, supporting screening,
epitope
binding, and relative potency assessment.
Selectivity profiling and translational ex vivo studies using patient-derived tissues and immune cell
functional
analysis further strengthen biological relevance and downstream development confidence.
In vitro screening and biochemical profiling services include:
- Primary biochemical screening assays across enzyme activity and affinity binding formats
- High-throughput technology platforms including fluorescence, luminescence, HTRF, AlphaLISA, absorbance, and calcium flux assays
- Secondary functional cell-based assays for cytotoxicity, proliferation, phosphorylation, cytokine release, and mechanistic pathway validation
- Quantitative SPR-based interaction analysis for kinetics, affinity determination, epitope binding, and relative potency profiling
- Selectivity assessment across isoforms and species, alongside ex vivo translational studies using patient-derived tissues and immune cell functional profiling
Talk to Our Experts
In Vitro Pharmacology
PI Health Sciences discovery Biology provides integrated or isolated services (FFS) to support target validation, biochemical and biophysical screening, cell based functional analysis, target engagement, MoA exploration, and biomarker profiling. We develop bespoke assays with validation/qualification (QC), in a medium to high through-put format, to support the characterization of compounds throughout the lead candidate (PCC) drug selection process. Our scientists have extensive discovery experience with various targets and therapeutic modalities, including but not limited to small molecules, PROTACs, molecular glues, bicyclics, mAbs and engineered mAbs. Our functional and mechanistic cell-based assays support the screening of both small and large molecules for a variety of diseases, to improve translational success in vivo. Our team supports various therapeutic areas, including oncology/immuno-oncology, autoimmunity, immunology/inflammation, and neurologic disorders.
Small Molecule Screening
PI Health Sciences delivers high-throughput biochemical screening assays to support small molecule discovery across enzyme activity, binding behavior, and kinetic characterization. Our assay formats include fluorescence, absorbance, fluorescence polarization, luminescence, FRET/TR-FRET, AlphaScreen, and NanoBRET, scalable across 96, 384, and 1536-well platforms. We also provide specialized screening workflows for GPCR targets, including SPR/Biacore and FACS-based binding studies, along with functional readouts such as calcium flux, cAMP, β-arrestin, and IP-1 modulation. For targeted protein degradation modalities such as PROTACs, we support binding and ternary complex assays, protein degradation confirmation via WB/JESS, and ubiquitination profiling.
Large Molecule Screening
PI Health Sciences offers integrated large molecule screening services for peptides, monoclonal antibodies, and engineered biologics, enabling affinity determination, specificity confirmation, and functional binding characterization. Our platforms include SPR-based kinetic profiling using Biacore 8K, FACS-based binding assays, ELISA-driven specificity testing, and high-content flow cytometry workflows to support rapid progression of biologic candidates through early discovery and optimization.
Therapeutic Area Specific Cell-Based Functional Assays
PI Health Sciences develops disease-relevant, cell-based functional assay systems to support mechanism-of-action exploration and translational screening across key therapeutic areas. In autoimmunity and inflammation, our capabilities include immune cell activation and differentiation assays across T, B, NK, macrophage, dendritic cell, MDSC, and regulatory T-cell subsets, alongside cytokine profiling, mixed lymphocyte reactions, cytotoxicity systems, and antibody-mediated effector assays such as ADCC, CDC, and ADCP. In oncology, we support cytotoxicity and proliferation workflows using platforms such as Incucyte, angiogenesis and apoptosis assays, motility and invasion studies, phosphorylation pathway analysis via WB/JESS, and comprehensive cell cycle and cell death profiling. Immuno-oncology assays extend into tumor microenvironment characterization through immune cell population mapping, checkpoint marker evaluation, and immune-cancer co-culture functional systems. For fibrosis, we provide epithelial-mesenchymal transition models and biomarker-linked gene expression validation by qPCR.
Ex Vivo Immunophenotyping and Immune Profiling
To enhance translational relevance, PI Health Sciences offers ex vivo immune profiling platforms leveraging patient-derived tissues, lymphoid samples, and peripheral blood systems. Our immunophenotyping workflows include deep flow cytometry-based subset analysis across CD4/CD8 populations, NK cells, B cells, dendritic cells, MDSCs, and macrophage polarization states. We evaluate immune exhaustion, activation, and checkpoint markers including PD-1, TIM-3, LAG-3, OX40, and 4-1BB, alongside cytokine and chemokine release profiling through ELISA and intracellular flow cytometry. Additional translational capabilities include lymphoid tissue response assessment and T cell dependent antibody response (TDAR) studies to support immune-functional validation across discovery programs.
Talk to Our Experts
Organoids
At PI Health Sciences , organoid-based translational platforms enable physiologically relevant disease modeling and predictive drug response assessment beyond conventional 2D systems. Our 3D organoid capabilities bridge discovery and translational biology by capturing tissue-level complexity, cellular heterogeneity, and clinically meaningful functional responses. We establish scalable organoid models to support mechanism exploration, therapeutic screening, and patient-relevant validation across key disease areas.
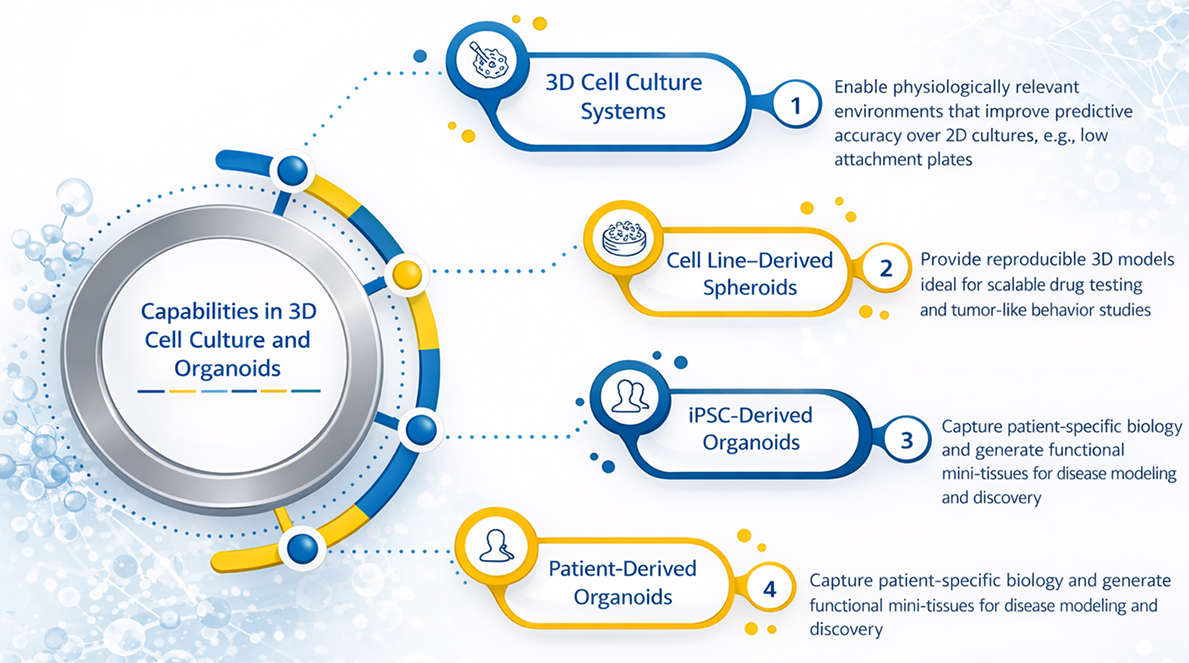
Advanced 3D cell culture and organoid platforms—including spheroids, iPSC-derived, and patient-derived organoids—enable physiologically relevant, scalable, and patient-specific models for improved drug testing and disease discovery.
Organoid and 3D translational biology services include:
- 3D cell culture systems for improved predictive accuracy over traditional monolayer assays
- Cell line-derived spheroids for reproducible, high-throughput drug testing and tumor-like behavior studies
- iPSC-derived organoids to model patient-specific biology and generate functional mini-tissues for disease discovery
- Patient-derived organoids (PDOs) to enable clinically aligned response profiling, personalized therapeutic evaluation, and translational biomarker development
Talk to Our Experts
Large Molecules
At PI Health Sciences , we provide specialized Discovery Biology workflows for the screening and characterization of large molecule therapeutics, including monoclonal antibodies (mAbs), antibody drug conjugates (ADCs), and bi-specific platforms. Our integrated screening cascade is designed to generate early, decision-ready evidence across binding performance, affinity strength, internalization behavior, and immune-mediated functional activity.We combine scalable assay formats with mechanistic and translational relevance, enabling confident selection and progression of biologic candidates across diverse therapeutic programs.
Binding Activity Assessment
PI Health Sciences initiates large molecule screening through robust binding activity assays to confirm antigen engagement and functional targeting. Our platforms support both recombinant antigen-based and cell-based binding evaluation.
Key capabilities include:
- Sandwich ELISA binding assays using Fc-tagged recombinant antigens
- FACS-based binding analysis using antigen-overexpressing CHO cell systems
- Binding assessment across varying expression levels (high, medium, low)
- Bi-specific binding assays across both tumor antigen and T-cell arms
Affinity Determination and Kinetic Profiling
To advance screening hits, we perform quantitative affinity determination using Biacore-based kinetic analysis. These studies provide critical insights into interaction strength and binding dynamics.
Services include:
- SPR-based affinity characterization of lead molecules
- Association and dissociation kinetic profiling
- Comparative ranking of candidate antibodies and constructs
Internalization and ADC Functionality Assays
For ADC and targeted biologic modalities, PI Health Sciences conducts internalization assays to quantify cellular uptake and target-driven internal trafficking, supporting payload delivery evaluation.
Capabilities include:
- High-throughput MMAF internalization assays
- Internalization kinetics using pH-sensitive dye methodologies
- Cellular uptake profiling to support ADC optimization
Immunology and Bispecific Functional Assays
For bi-specific molecules, screening extends into immune-mediated functional biology, enabling evaluation of T-cell redirection, conjugation formation, and target-specific cytotoxicity.
Key assays include:
- Bi-specific mediated T-cell and target cell conjugation analysis
- Cytotoxicity profiling in relevant co-culture systems
- Specificity assessment through selective effector cell depletion studies
Translational Extension and Cell Line Validation
PI Health Sciences further supports translational progression by extending cytotoxicity and functional assays across diverse suspension and adherent cell line models, ensuring activity consistency across clinically relevant contexts.
Applications include:
- Functional validation across multiple tumor and immune cell origins
- Translational relevance assessment beyond engineered systems
- Lead optimization support through expanded cell-based profiling
Throughput and Deliverables
Our large molecule screening platforms are scalable across:
- 24, 96, and 384-well assay formats
- Approx. ~100 molecules per week per FTE
Deliverables include:
- Percent inhibition
- Dose-response relationships
- IC₅₀ determination
- Binding, affinity, and internalization profiling
- Functional immune-mediated activity assessment
Talk to Our Experts
Therapeutic Areas
PI Health Sciences Discovery Biology delivers end-to-end assay expertise across key therapeutic areas,
supporting integrated drug discovery from early screening through translational validation. Our
platforms span
biochemical, cellular, genomic, proteomic, and advanced tumor model systems, enabling robust evaluation
of small
molecules, peptides, monoclonal antibodies, bispecifics, engineered antibodies, and emerging modalities.
We support oncology and immuno-oncology programs through comprehensive cytotoxicity, immune-mediated
killing,
checkpoint biology, and tumor microenvironment profiling workflows. Our immunology capabilities extend
into
autoimmune and inflammatory disease models, with deep expertise in immune cell activation,
differentiation,
cytokine profiling, and ex vivo immunophenotyping. Translational sciences are integrated through access
to
patient-derived tissues, peripheral blood, biopsies, and functional immune response characterization,
strengthening clinical relevance across discovery pipelines.
Assays
Biochemical Assay Expertise
- Kinase Activity Assays (ADP-Glo™)
- Binding and Selectivity Assays (TR-FRET, HTRF)
- Biophysical Interaction Assays (SPR, Fluorescence Polarization)
- Quantification Assays (AlphaLISA®, LANCE®)
Genomic and Proteomic Assays
- Gene Expression (qPCR, Microarray)
- Protein Expression (Western Blot, Immunofluorescence)
- Gene Silencing (siRNA/shRNA)
Analytical, In Vivo and Translational Models
- In Vivo Tumor Models (SCID Xenograft)
- Combination and Washout Studies
Ex Vivo and Translational Studies
- Immunophenotyping of TILs
- Activation Markers and Cytokine Profiling
- Transcriptome Analysis (NGS)
- Patient Tissue and Peripheral Blood Functional Immune Analysis
Immunology Assays
- Dedicated Functional Assays for:
- T Cells (Th1, Th2, Th17, CD8, NK Cells)
- Tregs
- MDSCs
- Macrophages
- Memory T Cells (CM, EM, EMRA)
- Dendritic Cells
- Immune Cell Stimulation
- Cytokine Production
- Proliferation Assays
- Antigen-Specific T Cell Recall Responses (PBMC and Animal Models)
Cellular Functional Assay Expertise
- Cell Viability and Apoptosis Assays (CellTiter-Glo®, Annexin V/PI-FACS)
- ADCC
- ADCP
- CDC
- Target Engagement Assays (HiBiT-LgBiT, NanoBRET)
Advanced Tumor Models and 3D Assays
- Amyloid-Based Hydrogels (3D Tumor Spheroid Formation)
- Biomarker Visualization (IF Microscopy)
- Live Imaging (Proliferation, Migration, Drug Response)
Immuno-Oncology In Vitro Assays
- Immune Cell Mediated Killing Assays (Small molecules, Bispecifics, Engineered Abs)
- Binding Studies (BIACORE, FACS)
- Effector:Target Co-culture Cytotoxicity Assays
- Immune Activation Marker Analysis (FACS, qPCR, NGS)
- Cytokine Release (Multiplex/Luminex)
- ADCC, ADCP
- Checkpoint Inhibitor Evaluation
- Mixed Lymphocyte Reaction (MLR)
- Biosimilar Evaluation
Talk to Our Experts
Key Strength

Therapeutic Expertise
Expertise across major disease areas including oncology, immuno-oncology, autoimmunity, inflammation, neurosciences, and fibrosis.
Laboratory & Compliance
GLP-like laboratories with plate-reader instruments compliant with 21 CFR Part 11 and computer system validation (CSV) to ensure data quality, integrity, and traceability for IND-ready submissions.

Target Classes
Comprehensive capabilities across GPCRs, kinases, phosphatases, proteases, and ion channels.
Therapeutic Modalities
Support for small molecules, PROTACs, AUTOTACs, molecular glues, and large molecules including monoclonal and engineered antibodies (in-vitro screening only).
Assay Platforms
Biochemical, biophysical, and primary cell-based phenotypic assay platforms enabling robust target and mechanism evaluation.
In-Vitro Screening Technologies
96- and 384-well screening formats covering absorbance, fluorescence, luminescence, FRET, HTRF, AlphaELISA, chemiluminescence, NanoBRET, LC-MS, and binding assays.


Imaging Capabilities
Advanced high-content imaging systems for detailed cellular and phenotypic analysis.
Frequently asked questions
We’re here to help with any questions you have about our plans, supported features, and how our model works.
What stages of drug discovery are supported by our Discovery Biology Services at PI Health Sciences?
Our Discovery Biology Services supports programs from early target identification and validation through hit-to-lead and candidate selection. Our services are designed to generate biologically robust evidence that enables confident transition into preclinical development.
What therapeutic modalities are covered under Discovery Biology services?
We support a broad range of modalities, including small molecules, PROTACs, molecular glues, and large molecules such as monoclonal and engineered antibodies, with in vitro biology and pharmacology capabilities tailored to each modality.
How does Discovery Biology integrate with chemistry and DMPK programs?
Our Discovery Biology teams operate in tight alignment with discovery chemistry and DMPK, ensuring biological feedback directly informs molecular design, SAR interpretation, and early liability assessment.
How does PI Health Sciences ensure biological data is translationally relevant?
We prioritize disease-relevant cellular systems, functional readouts, and biomarker strategies that reflect human biology, enabling clearer linkage between in vitro findings and downstream in vivo or clinical hypotheses.
Can Discovery Biology programs be customized to specific disease areas?
Our biology platforms are adaptable across oncology, immuno-oncology, autoimmunity, inflammation, neuroscience, and fibrosis, with disease-specific assay design and expert scientific oversight.
Contact Us
Connect with PI Health Sciences to explore how our discovery biology expertise advances target-centric programs through functional validation and biologically informed decision-making.
