LYTAC Platform

Lysosomal Targeting Chimeras (LYTAC) for Extracellular and Membrane Protein Degradation
At PI Health Sciences, our LYTAC (Lysosomal Targeting Chimera) platform enables targeted degradation of extracellular and membrane-bound proteins, expanding therapeutic reach beyond traditional intracellular targets. As a next-generation New Modalities approach, LYTAC degraders leverage endogenous lysosomal trafficking pathways to achieve complete and selective protein elimination rather than transient inhibition.
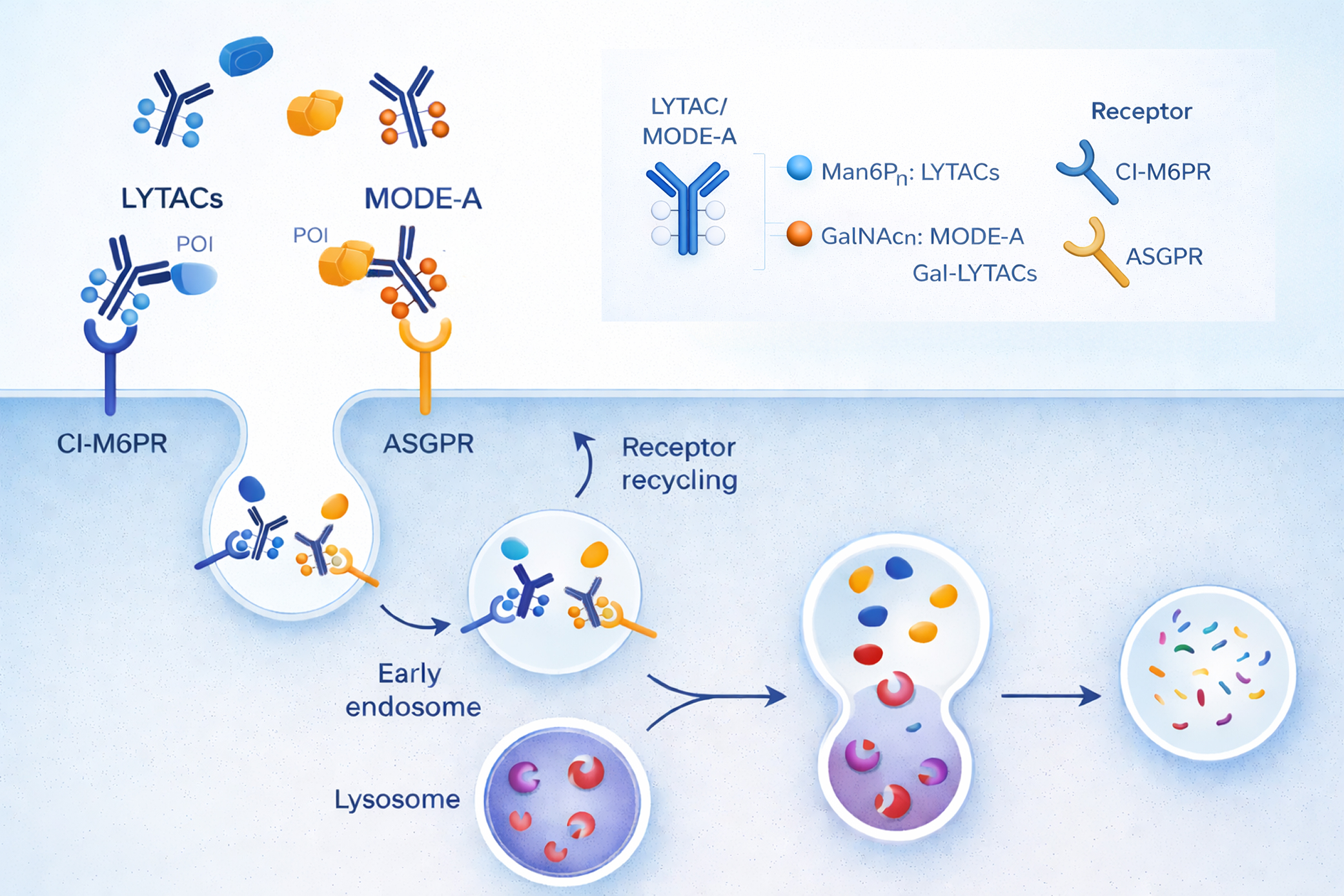
Our Lytac platform is designed to support programs from hit identification through preclinical candidate nomination, integrating target biology, receptor biology, glycochemistry, and antibody or ligand engineering under a single scientific framework. By exploiting cell-surface lysosome-targeting receptors, we enable access to disease-driving proteins previously considered undruggable, including receptor tyrosine kinases, immune checkpoint proteins, and pathogenic secreted factors.
By combining modular degrader design, receptor-specific tissue targeting, and mechanistic degradation readouts, PI Health Sciences delivers LYTAC programs that are translationally relevant, tissue-aware, and aligned with downstream IND-enabling expectations.
Hit-to-Candidate Workflow
Lysosome-Targeting Receptors (LTRs)
Validated LYTAC Targets & Therapeutic Areas
Our Capabilities
LYTAC Target Biology and Validation
Comprehensive validation of extracellular and membrane protein targets through receptor expression profiling, tissue distribution analysis, and degradability assessment to ensure biological relevance and translational feasibility.
Modular LYTAC Design and Engineering
Rational design of LYTAC degraders using antibodies, Fab fragments, peptides, or small-molecule binders, coupled with optimized lysosome-targeting receptor ligands for tissue-aware precision.
Glycochemistry and Conjugation Excellence
Advanced glycopeptide synthesis and site-specific conjugation technologies inspired by ADC platforms, enabling stable, reproducible LYTAC constructs with controlled stoichiometry.
Mechanism-Driven Degradation Assessment
End-to-end validation of ternary complex formation, receptor-mediated internalization, lysosomal trafficking, and quantitative degradation kinetics to confirm true protein elimination.
PK, PD, and In Vivo Translational Optimization
Integrated PK, tissue distribution, and exposure–degradation relationship studies with in vivo efficacy and selectivity profiling to support confident translational decision-making.
Preclinical and IND-Enabling Advancement
LYTAC programs developed with immunogenicity assessment, formulation strategy, GLP toxicology planning, CMC readiness, and regulatory alignment to enable seamless progression to IND submission
Frequently asked questions
We’re here to help with any questions you have about our plans, supported features, and how our model works.
What differentiates LYTAC degraders from other targeted protein degradation approaches?
LYTAC degraders enable lysosomal degradation of extracellular and membrane-bound proteins by hijacking cell-surface lysosome-targeting receptors. Unlike intracellular degradation strategies, LYTAC technology expands the druggable proteome to include secreted proteins, immune checkpoints, and receptor tyrosine kinases.
How does PI Health Sciences approach tissue-specific targeting in LYTAC programs?
Tissue specificity is achieved through receptor selection and ligand engineering, including ASGPR for liver targeting, TfR for CNS access, and CD206 for immune and microglial cells. Receptor expression and biodistribution are evaluated early to guide degrader design.
What protein formats can be used to build LYTAC degraders?
PI Health Sciences supports modular LYTAC design using monoclonal antibodies, Fab fragments, peptides, and small molecules as protein-of-interest binders, enabling flexibility across therapeutic areas and development stages.
How are LYTAC programs advanced toward IND readiness?
LYTAC candidates are progressed through integrated degradation validation, PK and tissue distribution studies, immunogenicity assessment, formulation development, and GLP-aligned toxicology planning, ensuring a coherent regulatory narrative for IND submission.
Contact Us
Connect with PI Health Sciences to explore how our LYTAC approach enables lysosome-directed degradation of extracellular and membrane proteins, spanning rational ligand design through translational preclinical evaluation.
