Toxicology Services
Home  Solutions
Solutions  Discovery
Discovery


Toxicology Services
Home  Solutions
Solutions  Discovery
Discovery
Toxicology Services for Safe & Compliant Drug Development
At PI Health Sciences, we deliver expert-led pharmaceutical toxicology services to ensure the safety, compliance, and efficacy of your drug development programs. Our toxicology solutions are designed to support early-stage discovery through regulatory submissions, backed by a strong scientific foundation and strategic partnerships with Contract Research Organizations (CROs), subject matter experts, and experienced toxicologists.
Whether you’re preparing for IND, NDA, or global submissions, our toxicology services streamline the process from protocol design to high-quality final reports.

Our Toxicology Services Model
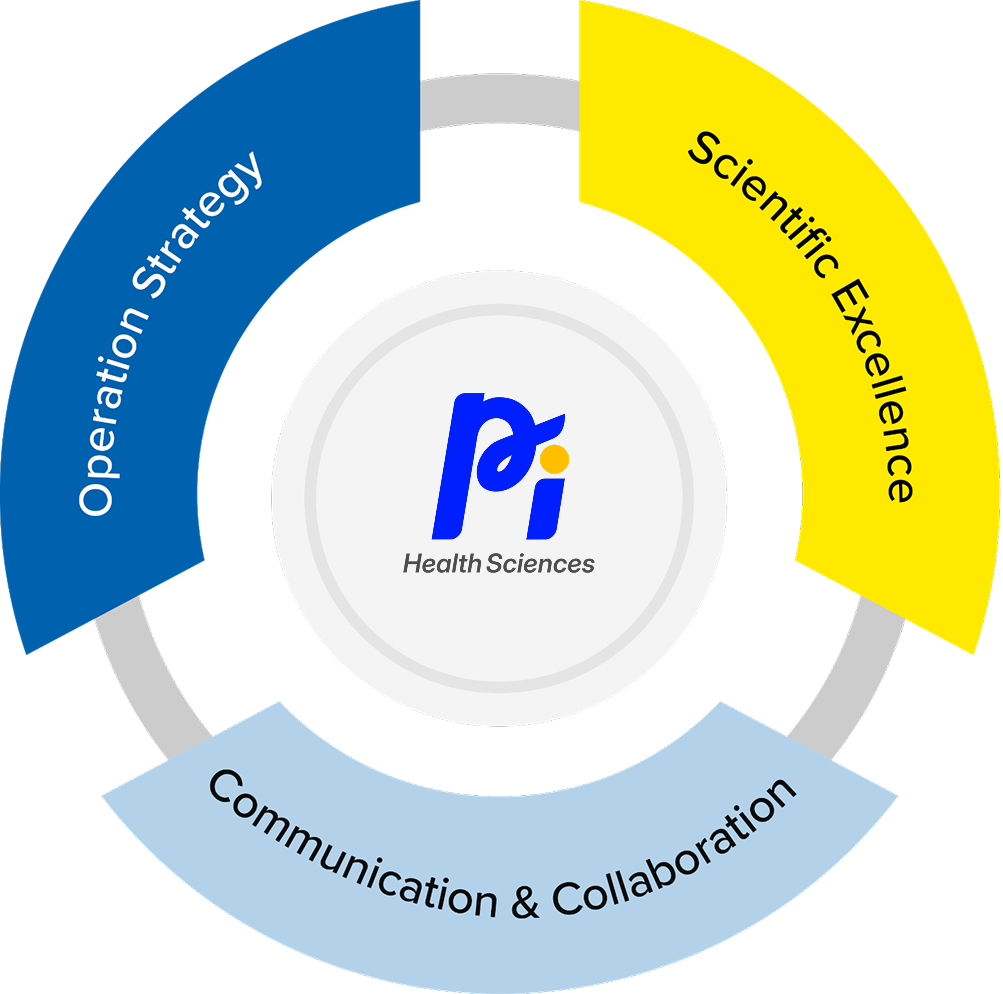
Operation Strategy:
-
Programs led by senior Study Directors who manage protocol development, dose range finding, and full study execution.
-
Expertise in species selection, route and method of administration, dose level design, and formulation planning.
-
Alignment with Good Laboratory Practice (GLP) and international regulatory standards ensures that all toxicology studies meet compliance requirements.
Scientific Excellence:
-
Protocols and study plans built on current global regulatory guidelines.
-
Comprehensive toxicology data analysis to extract meaningful, actionable safety insights.
-
Execution of both non-GLP dose range finding studies and GLP-compliant toxicology studies tailored to support regulatory approvals.
-
Preparation of high-quality submission dossiers—ready for IND, CTA, or NDA filing.
Transparent Collaboration:
-
Clear, frequent client communication to align on objectives throughout the study lifecycle.
-
Strategic refinements based on interim toxicological findings and evolving study needs.
-
A collaborative team approach that integrates your feedback into every phase of the toxicology services process.
Why Partner With Us?

End-to-End Integration
From study design to data reporting and submission dossier preparation.

Experienced Toxicologists
Our expert team brings decades of experience in preclinical toxicology.
Regulatory Expertise
Deep understanding of regional and global frameworks (FDA, EMA, etc.).

Client-Centric Approach
We operate as an extension of your team, ensuring agile, responsive study execution.
Scientific Rigor
High standards for accuracy, repeatability, and data integrity.
Facilities & Capabilities

Dose Range Finding Studies
Non-GLP preliminary studies to define safe and effective dose levels.

GLP-Compliant Toxicology Studies
Comprehensive toxicological assessments following regulatory guidelines.

Regulatory Submissions
High-quality dossiers to facilitate swift regulatory approval.

Collaborative Networks
Partnerships with leading CROs and toxicology experts to deliver tailored solutions.
Contact Us
Partner with PI Health Sciences for expert-led toxicology services that ensure the success of your drug development programs. Reach out today to explore how our pharmaceutical toxicology services can support your journey towards safe and compliant drug development.expertise can support your drug development program.
