Integrated Flow Chemistry Services

Revolutionizing Pharmaceutical Manufacturing with Advanced Flow Chemistry
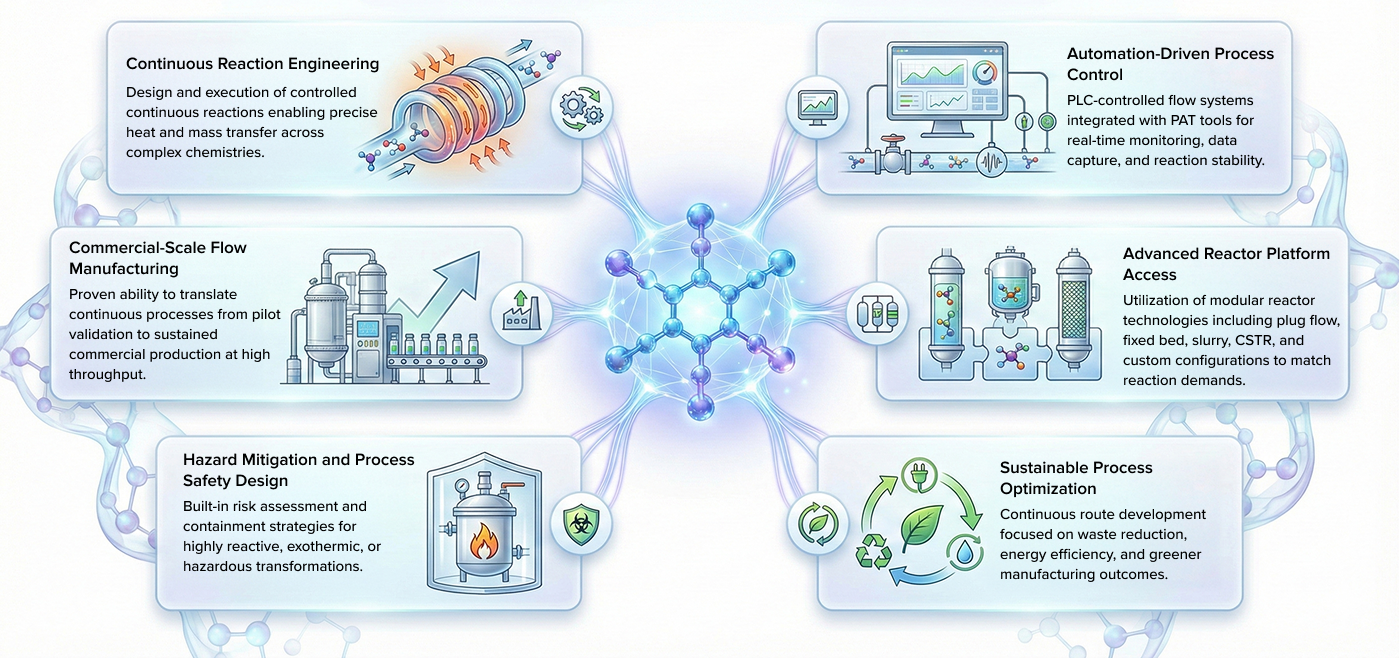
At PI Health Sciences, flow chemistry in the pharmaceutical industry integrates automation, process optimization, and advanced data systems to enable continuous manufacturing with improved scalability and safety. Designed to enhance process efficiency and greenness, it bridges innovation and sustainability through automated pilot plants, advanced flow reactors, and a dedicated multidisciplinary team. Our flow chemistry services bring together critical domains of Process Safety, Particle Science & Engineering, and Lab Automation to create a seamless ecosystem of high-precision chemical transformation. Leveraging automated R&D systems and PAT tools, Flow Chemistry ensures real-time data acquisition, optimized reaction control, and process stability.
By combining reactive hazard assessment, real-time monitoring, and modular flow systems, PI Health Sciences accelerates product development, supports greener synthesis, and enables the transition from batch to continuous operation with uncompromised quality. Through our flow chemistry services, we empower pharmaceutical innovators to scale up with confidence, achieve commercial readiness, and maintain sustainability at every stage of manufacturing. With advanced reactor technologies, automation-enabled pilot plants, and a multidisciplinary engineering team, PI Health Sciences continues to redefine how flow chemistry in the pharmaceutical industry drives efficiency, precision, and long-term value creation.
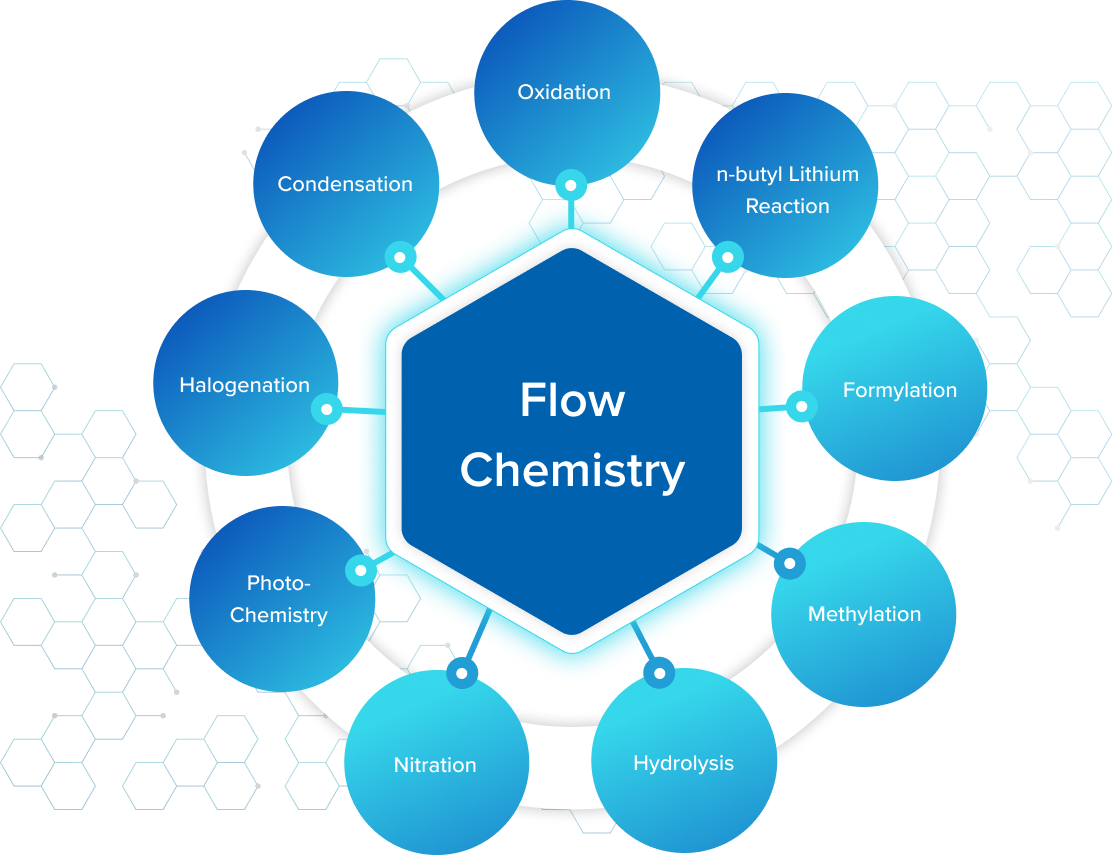
Our Flow Chemistry Model
Our Capabilities
Frequently asked questions
We’re here to help with any questions you have about our plans, supported features, and how our model works.
What types of pharmaceutical reactions are suitable for flow chemistry at PI Health Sciences?
Our flow chemistry platform supports a wide range of reactions including highly exothermic, hazardous, gas–liquid, slurry-based, and multistep transformations. Validated examples include formylation, chlorination, oxidation, and vapour-phase fluorination, all executed under controlled, continuous conditions.
How does flow chemistry improve safety compared to traditional batch manufacturing?
Flow chemistry minimizes reactive inventory, enables precise control of reaction parameters, and incorporates real-time monitoring through PAT tools. This significantly reduces thermal and reactive hazards while ensuring consistent product quality throughout the process.
Can flow chemistry processes be scaled to commercial manufacturing volumes?
Yes. Our flow chemistry services are designed with scalability in mind, enabling seamless transition from laboratory development to pilot-scale validation and commercial production, with demonstrated capacities of up to 8–10 MT per day.
How does PI Health Sciences support the transition from batch to continuous manufacturing?
We follow a structured approach that combines reactive hazard assessment, process modeling, automated pilot studies, and real-time data integration. This ensures smooth conversion from batch processes to continuous flow systems without compromising quality, regulatory compliance, or supply reliability.
Insights & Resources
Insights from across our services
Contact Us
Unlock the full potential of Flow Chemistry with PI Health Sciences.
Get in touch with our experts today to discover tailored Flow Chemistry Services designed to accelerate and optimize your pharmaceutical processes.