In Silico Genotoxicity

Regulatory-Ready Decisions from Discovery to Development
The In Silico Genotoxicity Assessment Platform at PI Health Sciences is built to remove ambiguity from early safety decisions. Instead of discovering genotoxic liabilities late, when chemistry options are limited and timelines are on fire, this platform enables proactive, data-driven risk evaluation at the point where change is still cheap and smart. In silico genotoxicity assessment has evolved from a supportive tool into a regulatory-anchored decision system, particularly under ICH M7(R2). At its core, it allows teams to predict and contextualize mutagenic risk before committing to synthesis, scale-up, or experimental testing. The result is fewer surprises, fewer dead ends, and tighter alignment between chemistry strategy and regulatory expectations.
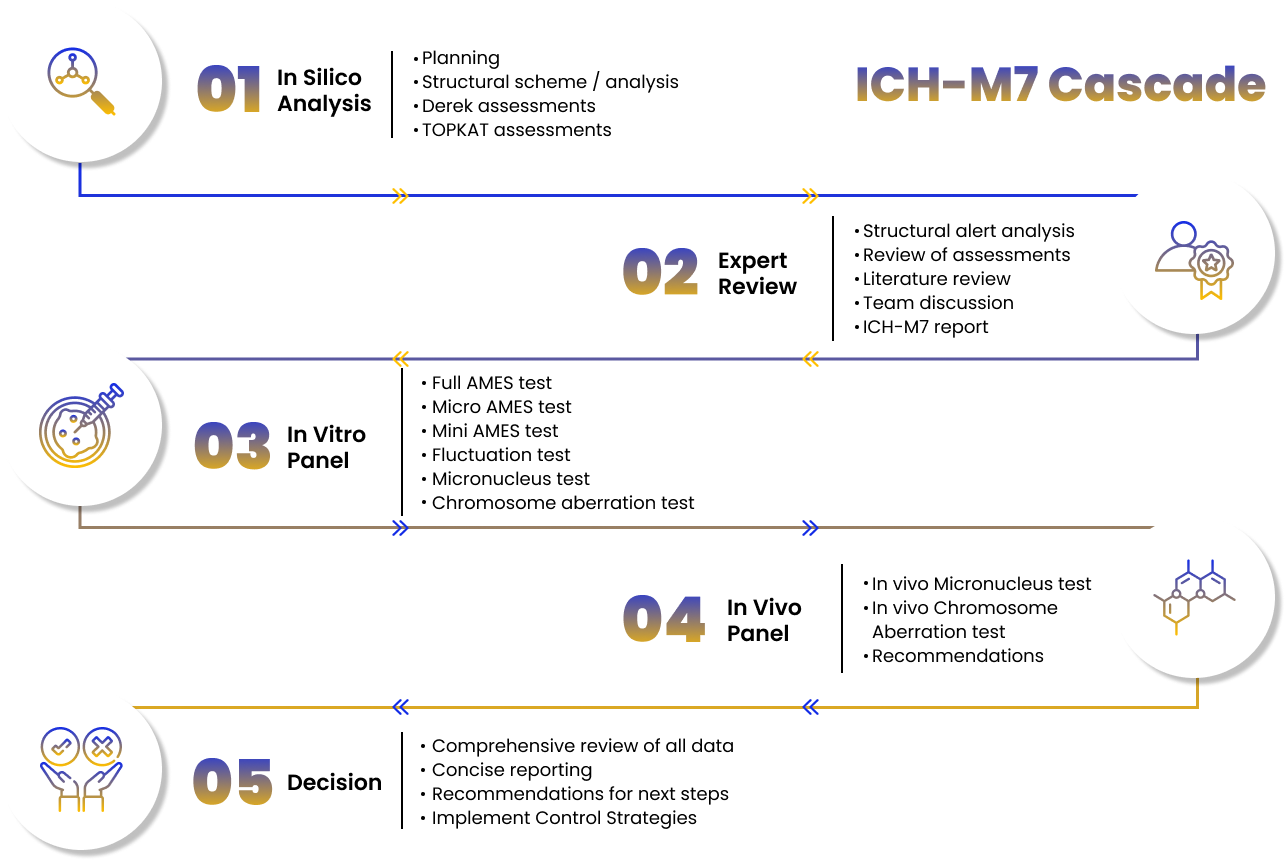
Trusted by pharmaceutical and biotechnology companies, CROs, CDMOs, and safety consultants, our in silico genotoxicity platform supports regulatory decision making across drug substances, drug products, impurities, degradants, intermediates, excipients, and related chemicals within modern toxicology frameworks.
The platform aligns fully with ICH M7 requirements, applying two complementary (Q)SAR methodologies, one expert rule based and one statistical or machine learning driven. All predictions are reviewed by qualified scientists and integrated into a clear, defensible weight of evidence narrative. Deliverables are submission ready and include applicability domain assessment, structural alert rationale, uncertainty handling, and transparent impurity classification logic, ensuring confident regulatory acceptance and actionable outcomes.
Our Capabilities
ICH M7(R2)–Aligned Scientific Governance
End-to-end in silico genotoxicity assessments executed using two complementary (Q)SAR methodologies, one expert rule-based and one statistical or ML-driven, fully aligned with ICH M7 and OECD principles for global regulatory acceptance.
Regulatory-Ready Mutagenicity & Impurity Risk Assessment
Ames-equivalent mutagenicity prediction and evaluation of drug substances, impurities, degradants, and intermediates, including structural alert analysis, applicability domain checks, and ICH M7-style impurity classification.
Expert-Led Weight-of-Evidence Interpretation
Human toxicology expertise layered on AI outputs to resolve conflicting or borderline predictions, assess mechanistic plausibility, and translate model signals into clear, defensible regulatory conclusions.
Submission-Quality Documentation & Reporting
Transparent, submission-ready reports linking structure, prediction, uncertainty, and classification logic, designed for direct inclusion in regulatory dossiers and efficient authority review.
Development-Stage Continuity & Integration
Outputs structured to remain usable from early discovery through development, supporting coherent chemistry, toxicology, CMC, and client-led regulatory workflows.
Flexible, Secure Engagement Models
Confidential and scalable delivery, ranging from single-impurity questions to portfolio-level screening programs across pharmaceutical and chemical pipelines.
Frequently asked questions
We’re here to help with any questions you have about our plans, supported features, and how our model works.
When should in silico genotoxicity assessment be applied during drug development?
In silico genotoxicity assessment is most impactful during hit-to-lead and early optimization, when chemical redesign is still feasible and cost-effective. PI Health Sciences helps teams apply these assessments early to flag mutagenic liabilities before synthesis scale-up or experimental testing, enabling faster iteration, smarter impurity control strategies, and fewer late-stage surprises.
How does PI Health Sciences support impurity risk decisions under ICH M7?
PI Health Sciences goes beyond running models by actively guiding impurity risk strategy. We assess process-related impurities and degradants, classify them under ICH M7, and provide clear scientific justification that supports acceptable intake limits, control strategies, or the need for follow-up testing. This allows chemistry and CMC teams to make confident, regulator-aligned decisions without over-testing.
Can in silico genotoxicity results reduce or avoid experimental testing?
Yes, when used appropriately. Well-documented negative (Q)SAR assessments interpreted by experts can often remove the need for immediate in vitro genotoxicity studies, especially for impurities. PI Health Sciences ensures predictions are supported by applicability domain analysis and weight-of-evidence rationale, increasing the likelihood of regulatory acceptance and reducing unnecessary experimental burden.
Are the outputs suitable for later regulatory submissions and inspections?
Absolutely. PI Health Sciences structures all reports to remain usable beyond early discovery, with clear traceability from chemical structure to final conclusion. This continuity supports client-led regulatory submissions, responses to authority questions, and internal audits, without the need to recreate or reinterpret earlier genotoxicity assessments.
Insights & Resources
Insights from across our services
Contact Us
Connect with PI Health Sciences to explore how our customized pharmaceutical development services can streamline your molecule’s journey from concept to market with precision and confidence.

